The Vaccine Is Here
The COVID-19 Vaccine
The world has been dealing with COVID-19 for about a year now. Businesses shut down, curfews were put into place, masks have been mandatory. The United States has recorded over 25 million cases and over 400,000 deaths, making up almost one-third of the global recorded cases. Hope has been restored, though, with the development of the COVID-19 vaccine. The vaccine was worked on for most of 2020, but scientists already had most of the research done. SARS-CoV-2, a strain of the coronavirus, started an epidemic in 2002, and scientists have been developing a vaccine for that for years. With increased funding and knowledge of the virus, a vaccine was able to be developed in a short amount of time.
Typically, vaccines can take years to develop. The quickest vaccine to be developed before COVID-19 was the mumps vaccine, which took 4 years. Some vaccines are made in hen’s eggs, including the influenza vaccine developed in the 1930s after the flu pandemic in 1918-1920. With eggs, they are able to put the virus into a fertilized egg and leave it for several days so it can multiply. It’s then harvested from the egg and purified and killed.
But scientists have developed vaccines using mRNA technology. Scientists use a copy of RNA to build an immune response. Once the vaccine is in the body, the RNA acts as mRNA, which is the messenger for RNA, and protein is built and the body learns the immune response.
I met with a doctor (for anonymity sake we’ll call him Dr. Mayfield), who distributes vaccines, and talked about the current situation with COVID-19 vaccines. “I can assure everyone that the COVID vaccine is completely safe,” he said. “I’ve been giving vaccines out for years now and I haven’t had any troubles.” I asked him about mRNA technology, and he replied “It’s nothing new. Scientists have developed vaccines using mRNA and have done plenty of research.”
Both Pfizer and Moderna have developed a vaccine for COVID-19. Pfizer is an American pharmaceutical company, founded in 1849 in New York. Moderna, a biotechnology company in Massachusetts, worked with the National Institute of Allergy and Infectious Diseases and the Biomedical Advanced Research and Development Authority to develop the COVID-19 vaccine. Both vaccines use mRNA technology, and none of them contain eggs, latex, and preservatives.
Moderna’s ingredients are messenger ribonucleic acid (mRNA), lipids (SM-102, polyethylene glycol [PEG] 2000 dimyristoyl glycerol [DMG], cholesterol, and 1,2-distearoyl-sn-glycero-3-phosphocholine [DSPC]), tromethamine, tromethamine hydrochloride, acetic acid, sodium acetate, and sucrose. Pfizer’s vaccine contains mRNA, lipids ((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate), 2 [(polyethylene glycol)-2000]-N,N-ditetradecylacetamide, 1,2-Distearoyl-sn-glycero-3- phosphocholine, and cholesterol), potassium chloride, monobasic potassium phosphate, sodium chloride, dibasic sodium phosphate dihydrate, and sucrose. Moderna’s vaccine takes two doses 28 days apart, while Pfizer’s takes two doses 21 days apart. Both vaccines have mild side effects, including pain, swelling, headaches, and chills, but some more severe side effects have occurred due to allergies. It’s best to talk to your doctor if you are allergic to any of the ingredients before getting the vaccine.
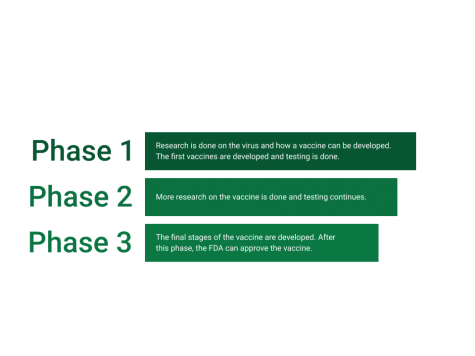
The COVID-19 vaccine was developed in three phases, where testing was done in each one. After phase three, the FDA has to approve the vaccine for emergency use by reviewing the data and research done. Pfizer’s vaccine was approved on December 12, and Moderna’s was approved on December 18.
One of the greatest outcomes of the COVID-19 testing was the number of people who volunteered. More than 100,000 people volunteered for testing during the vaccine trials. This is the most volunteers for a vaccine test ever recorded in history. Most people say it’s due to the seriousness of the situation and the high funding for the research. According to the CDC, 79.4% of the volunteers who were tested during Moderna’s trials were white, 20% were Hispanic, and 9.7% were black. Similar results came with the Pfizer trials, with 81.9% white volunteers, 26.2% Hispanic volunteers, and 9.8% black volunteers. The wide margin is due to the mistrust in medicine created in the black and Hispanic population, after forcefully doing tests on them over the years, such as in the Tuskegee Syphilis Experiments.
Right now, the people getting the COVID-19 vaccine are healthcare workers and long-term care facility workers. Cindy Schuster, a healthcare worker at Johns Hopkins Wellness Center, was one of the first to get the vaccine in Maryland. She was picked out of a number of people to get it and had the highest priority due to being on the frontlines during the pandemic.
“I feel like I won the lottery,” she stated. Cindy called the process “super smooth”, only having a sore arm afterward. She got the first dose of the vaccine on December 16th and got the second dose on January 4th. When asked why she got the vaccine, she said “I need to be an example for others, being a healthcare worker.”
In Maryland, the next people to get the COVID-19 vaccine are people who are 75 years old or older, inmates, teachers, and people living in special needs homes. Those vaccines will start being distributed at the end of January. Other vaccines are also on the way to being approved, including Dynavax, Sanofi, and Novavax.